Novel Oxygen Isotope Observed for the First Time

Scientists have made an intriguing discovery – a never-before-seen form of oxygen. This new isotope, called oxygen-28, has the highest number of neutrons ever observed in an oxygen atom’s nucleus. Despite expectations of stability, oxygen-28 actually decays rapidly, challenging our understanding of the “magic” number of particles in an atom’s nucleus.
The nucleus of an atom contains nucleons, which are subatomic particles made up of protons and neutrons. While the atomic number of an element is determined by its protons, the number of neutrons can vary, resulting in different isotopes. Oxygen, for example, has 8 protons but can have varying numbers of neutrons. Previously, the largest number of neutrons observed in an oxygen isotope was 18 in oxygen-26.
Oxygen-28: Unveiling a New Isotope
A team led by nuclear physicist Yosuke Kondo from Tokyo Institute of Technology has discovered two new oxygen isotopes, oxygen-27 and oxygen-28, with 19 and 20 neutrons, respectively. The team conducted their research at the RIKEN Radioactive Isotope Beam Factory, a facility designed to produce unstable isotopes using a cyclotron accelerator.
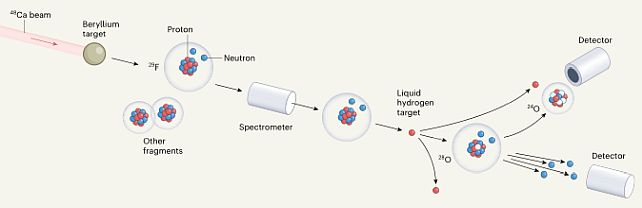
Creating Oxygen-28: The team used a two-step process to create the oxygen-28 isotope. First, they fired a beam of calcium-48 isotopes at a beryllium target to generate lighter atoms, including fluorine-29, which has 9 protons and 20 neutrons. They then collided this fluorine-29 with a liquid hydrogen target to remove a proton and create oxygen-28. The result was surprising – both oxygen-27 and oxygen-28 turned out to be unstable, quickly decaying into oxygen-24 and 3 or 4 loose neutrons, respectively.
The instability of oxygen-28 is intriguing because both 8 and 20 are considered “magic” numbers for protons and neutrons, respectively. These magic numbers indicate that the nucleus is expected to be stable. The stability of a nucleus depends on the filling of proton and neutron shells, which are energy levels that determine the stability of an atom. A nucleus in which both proton and neutron shells are completely filled is known as a doubly magic nucleus. Below is the diagram of the experiment (Nature):
Island of Inversion Hypothesis
Most of the oxygen on Earth, including the oxygen we breathe, is oxygen-16, which is doubly magic. Oxygen-28 was believed to be the next doubly magic isotope after oxygen-16, but previous attempts to find it were unsuccessful. The new findings by Kondo and his colleagues suggest that the neutron shell was not completely filled in oxygen-28. This challenges the assumption that 20 is a magic number for neutrons.
Interestingly, this inconsistency is consistent with a phenomenon known as the “island of inversion” for isotopes of neon, sodium, and magnesium, where shells of 20 neutrons are not fully closed. This also extends to fluorine-29 and, now, apparently oxygen-28. Further research is needed to understand the unclosed neutron shell, and investigating the nucleus in an excited, higher-energy state could provide more insights. Additionally, alternative methods of forming oxygen-28 could also offer valuable information, although they are more challenging to achieve.
Overall, the findings from Kondo’s team shed light on the complexity of doubly magic nuclei. This discovery challenges our previous understanding and opens up new avenues for exploring the mysteries of atomic structure. The research has been published in the journal Nature.